Ameliorative Effects of Supplements of Papaya Seed, Watermelon Seed, and Clove buds on Testosterone-DMBA Induced Prostate Cancer in Wistar Rats: Oxidative Stress, Inflammation, and Histological Analysis
Main Article Content
Abstract
Background: Prostate cancer is the second most common cancer among men worldwide with escalating incidence and mortality rates. This study investigated the protective effects of papaya seed, watermelon seed, and clove bud on testosterone-DMBA-induced prostate cancer in male Wistar rats.
Methods: Seventy male Wistar rats were randomly divided into seven groups: normal control, cancer control, combination supplementation (papaya seed, watermelon seed and clove bud, individual supplementations of papaya seed, watermelon seed, clove bud, and flutamide treatment. Prostate cancer was induced by single dose intraperitoneal injection of DMBA 65mg/kg) and subcutaneous testosterone (3mg/kg) continued for 12 weeks. The intervention groups received their respective supplementations 2 weeks before induction and continued after the induction. At the end of the intervention period, oxidative stress markers, inflammatory markers, and histopathological changes were assessed.
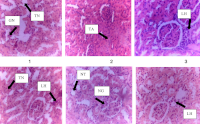
Results: Watermelon seed supplementation provided optimal balanced protection, significantly preserving catalase activity (6.87±0.63 vs. 4.67±1.17 µ/ml) and reducing CRP levels (2.70±0.25 vs. 12.47±5.17 mg/L). Clove bud supplementation effectively reduced nitric oxide levels (193.92±43.85 vs. 588.35±127.24 µm/mL) and IL-6 (31.47±4.24 vs. 34.88±4.03 pg/mL). Combination treatment demonstrated complete prostate and liver tissue protection. All dietary interventions provided complete hepatoprotection, contrasting with flutamide's hepatotoxicity. COX-2 levels were significantly lower in dietary groups (199.04±89.42 to 324.78±17.96 pg/mL) versus flutamide (613.07±201.71 pg/mL). Watermelon supplementation achieved complete renoprotection with preserved glomerular architecture.
Conclusion: Natural dietary supplements demonstrate organ-specific protective effects against prostate cancer with superior safety profiles compared to conventional treatment. These findings support their potential as supplementary therapeutic strategies for minimizing treatment-associated toxicities while enhancing outcomes.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0 International License.
The Journal is owned, published and copyrighted by the Nigerian Medical Association, River state Branch. The copyright of papers published are vested in the journal and the publisher. In line with our open access policy and the Creative Commons Attribution License policy authors are allowed to share their work with an acknowledgement of the work's authorship and initial publication in this journal.
This is an open access journal which means that all content is freely available without charge to the user or his/her institution. Users are allowed to read, download, copy, distribute, print, search, or link to the full texts of the articles in this journal without asking prior permission from the publisher or the author.
The use of general descriptive names, trade names, trademarks, and so forth in this publication, even if not specifically identified, does not imply that these names are not protected by the relevant laws and regulations. While the advice and information in this journal are believed to be true and accurate on the date of its going to press, neither the authors, the editors, nor the publisher can accept any legal responsibility for any errors or omissions that may be made. The publisher makes no warranty, express or implied, with respect to the material contained herein.
TNHJ also supports open access archiving of articles published in the journal after three months of publication. Authors are permitted and encouraged to post their work online (e.g, in institutional repositories or on their website) within the stated period, as it can lead to productive exchanges, as well as earlier and greater citation of published work (See The Effect of Open Access). All requests for permission for open access archiving outside this period should be sent to the editor via email to editor@tnhjph.com.
How to Cite
References
1. Schafer EJ, Laversanne M, Sung H, Soerjomataram I, Briganti A, Dahut W, Bray F, Jemal A. Recent patterns and trends in global prostate cancer incidence and mortality: an update. European Urology. 2025;87(3):302-313. Available from: https://doi.org/10.1016/j.eururo.2024.11.013.
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: A Cancer Journal for Clinicians. 2023;73(1):17-48. Available from: https://doi.org/10.3322/caac.21763
3. Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Letters. 2009;282(2):125-136. Available from: https://doi.org/10.1016/j.canlet.2008.12.011
4. Knudsen BS, Vasioukhin V. Mechanisms of prostate cancer initiation and progression. Advances in Cancer Research. 2010;109:1-50. Available from: https://doi.org/10.1016/B978-0-12-380890-5.00001-6
5. Murray TB. The pathogenesis of prostate cancer. Exon Publications; 2021. p. 29-41. Available from: https://doi.org/10.36255/exonpublications.prostatecancer.pathogenesis.2021
6. Shiota M. Oxidative stress and prostate cancer. In: Cancer. Academic Press; 2021. p. 15-26. Available from: https://doi.org/10.1016/B978-0-12-819547-5.00002-X
7. Testa U, Castelli G, Pelosi E. Cellular and molecular mechanisms underlying prostate cancer development: therapeutic implications. Medicines. 2019;6(3):82. Available from: https://doi.org/10.3390/medicines6030082
8. Cai C, Balk SP. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocrine-Related Cancer. 2011;18(5):R175-R182. Available from: https://doi.org/10.1530/ERC-10-0339
9. Chang KH, Li R, Papari-Zareei M, Watumull L, Zhao YD, Auchus RJ, Sharifi N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proceedings of the National Academy of Sciences. 2011;108(33):13728-13733. Available from: https://doi.org/10.1073/pnas.1107898108
10. Dai C, Heemers H, Sharifi N. Androgen signaling in prostate cancer. Cold Spring Harbor Perspectives in Medicine. 2017;7(9):a030452. Available from: https://doi.org/10.1101/cshperspect.a030452
11. Girling JS, Whitaker HC, Mills IG, Neal DE. Pathogenesis of prostate cancer and hormone refractory prostate cancer. Indian Journal of Urology. 2007;23(1):35-42. Available from: https://doi.org/10.4103/0970-1591.30265
12. Tan MH, Li J, Xu HE, Melcher K, Yong EL. Androgen receptor: structure, role in prostate cancer and drug discovery. Acta Pharmacologica Sinica. 2015;36(1):3-23. Available from: https://doi.org/10.1038/aps.2014.18
13. Wilczynski C, Agrawal L. Testosterone effects on the prostate gland: Review of pathophysiology and considerations in prostate cancer. Journal of Family Medicine and Disease Prevention. 2015;1(4). Available from: https://doi.org/10.23937/2469-5793/1510004
14. Bellamri M, Turesky RJ. Dietary carcinogens and DNA adducts in prostate cancer. In: Prostate Cancer: Cellular and Genetic Mechanisms of Disease Development and Progression. 2019. p. 29-55. Available from: https://doi.org/10.1007/978-3-030-32656-2_2
15. Katz ISS, Albuquerque LL, Suppa AP, da Silva GB, Jensen JR, Borrego A, et al. 7,12-Dimethylbenz(a)anthracene-induced genotoxicity on bone marrow cells from mice phenotypically selected for low acute inflammatory response. DNA Repair. 2016;37:43-52. Available from: https://doi.org/10.1016/j.dnarep.2015.11.006
16. Ma Z, Kim YM, Howard EW, Feng X, Kosanke SD, Yang S, et al. DMBA promotes ErbB2-mediated carcinogenesis via ErbB2 and estrogen receptor pathway activation and genomic instability. Oncology Reports. 2018;40(3):1632-1640. Available from: https://doi.org/10.3892/or.2018.6545
17. Singh R, Manna PP. Reactive oxygen species in cancer progression and its role in therapeutics. Exploration of Medicine. 2022;3(1):43-57. Available from: https://doi.org/10.37349/emed.2022.00073
18. Biesiadecki M, Mołoń M, Balawender K, Kobylińska Z, Galiniak S. Shedding light on the shadows: Oxidative stress and its pivotal role in prostate cancer progression. Frontiers in Oncology. 2024;14:1393078. Available from: https://doi.org/10.3389/fonc.2024.1393078
19. Duru R, Njoku O, Maduka I. Oxidative stress indicators in patients with prostate disorders in Enugu, South-East Nigeria. BioMed Research International. 2014;2014:313015. Available from: https://doi.org/10.1155/2014/313015
20. Erhabor O, Hussaini AM, Abdulwahab-Ahmed A, Retsky M, Erhabor T. Some oxidative stress biomarkers among patients with prostate cancer in Sokoto, North Western Nigeria. Open Journal of Blood Diseases. 2022;12(3):60-78. Available from: https://doi.org/10.4236/ojbd.2022.123007
21. Kim J, Mizokami A, Shin M, Izumi K, Konaka H, Kadono Y, et al. SOD3 acts as a tumor suppressor in PC-3 prostate cancer cells via hydrogen peroxide accumulation. Anticancer Research. 2014;34(6):2821-2831. Available from: https://doi.org/10.21873/anticanres.9748
22. Shukla S, Srivastava JK, Shankar E, Kanwal R, Nawab A, Sharma H, et al. Oxidative stress and antioxidant status in high-risk prostate cancer subjects. Diagnostics. 2020;10(3):126. Available from: https://doi.org/10.3390/diagnostics10030126
23. Ohtake S, Kawahara T, Ishiguro Y, Takeshima T, Kuroda S, Izumi K, et al. Oxidative stress marker 8-hydroxyguanosine is more highly expressed in prostate cancer than in benign prostatic hyperplasia. Molecular and Clinical Oncology. 2018;9(3):302-304. Available from: https://doi.org/10.3892/mco.2018.1665
24. Michalaki V, Syrigos K, Charles P, Waxman J. Serum levels of IL-6 and TNF-α correlate with clinicopathological features and patient survival in patients with prostate cancer. British Journal of Cancer. 2004;90(12):2312-2316. Available from: https://doi.org/10.1038/sj.bjc.6601814
25. Nguyen DP, Li J, Tewari AK. Inflammation and prostate cancer: the role of interleukin 6 (IL-6). BJU International. 2014;113(6):986-992. Available from: https://doi.org/10.1111/bju.12452
26. Ogbonnaya UC, Dappa BD. Comparative study of interleukin-6 (IL-6) and tumour necrosis factor alpha (TNF-α) levels in prostate cancer and benign prostatic hyperplasia subjects. Journal of Applied Health Sciences and Medicine. 2023;10. Available from: https://doi.org/10.58614/jahsm344
27. Situmorang PC, Ilyas S, Nugraha SE, Syahputra RA, Nik Abd Rahman NMA. Prospects of compounds of herbal plants as anticancer agents: a comprehensive review from molecular pathways. Frontiers in Pharmacology. 2024;15:1387866. Available from: https://doi.org/10.3389/fphar.2024.1387866
28. Bayala B, Zohoncon TM, Djigma FW, Nadembega C, Baron S, Lobaccaro JM, et al. Antioxidant and antiproliferative activities on prostate and cervical cultured cancer cells of five medicinal plant extracts from Burkina Faso. International Journal of Biological and Chemical Sciences. 2020;14(3):652-663. Available from: https://doi.org/10.4314/ijbcs.v14i3.1
29. Hao Q, Wu Y, Vadgama JV, Wang P. Phytochemicals in inhibition of prostate cancer: evidence from molecular mechanisms studies. Biomolecules. 2022;12(9):1306. Available from: https://doi.org/10.3390/biom12091306
30. Lekhak N, Bhattarai HK. Phytochemicals in cancer chemoprevention: preclinical and clinical studies. Cancer Control. 2024;31:10732748241302902. Available from: https://doi.org/10.1177/10732748241302902
31. Moriasi G, Ngugi M, Mwitari P, Omwenga G. In vitro anti-prostate cancer efficacy and phytochemical composition of the dichloromethane and ethyl acetate leaf extracts of Vitex doniana (sweet). Frontiers in Pharmacology. 2024;15:1483856. Available from: https://doi.org/10.3389/fphar.2024.1483856
32. Adeoye RI, Olopade ET, Olayemi IO, Okaiyeto K, Akiibinu MO. Nutritional and therapeutic potentials of Carica papaya Linn. seed: a comprehensive review. Plant Science Today. 2024;11(2):2348-900. Available from: https://doi.org/10.14719/pst.2843
33. Alotaibi KS, Li H, Rafi R, Siddiqui RA. Papaya black seeds have beneficial anticancer effects on PC-3 prostate cancer cells. Journal of Cancer Metastasis and Treatment. 2017;3:161-168. Available from: https://doi.org/10.20517/2394-4722.2017.33
34. Ebenyi LN, Ominyi MC, Anyanwu CB, Ogah O, Ogbanshi ME. The leaf extract of Carica papaya alleviates benign prostate hyperplasia in male Albino rats by antioxidative mechanisms. Nigerian Journal of Biochemistry and Molecular Biology. 2022;37(4):298-302.
35. Aderiye BI, David OM, Fagbohun ED, Faleye J, Olajide OM. Immunomodulatory and phytomedicinal properties of watermelon juice and pulp (Citrullus lanatus Linn): a review. GSC Biological and Pharmaceutical Sciences. 2020;11(2):153-165. Available from: https://doi.org/10.30574/gscbps.2020.11.2.0079
36. Ayubi N, Syafawi A, Padmasari DF, Putri DR, Komaini A, Yulfadinata A, et al. Anti-oxidant and anti-inflammatory properties of watermelon (Citrullus lanatus) have the potential to reduce oxidative stress and inflammation after exercise/physical activity: Systematic review. Retos. 2024;1(55):20-26. Available from: https://doi.org/10.47197/retos.v55.103029
37. Nissar J, Sidiqi US, Dar AH, Akbar U. Nutritional composition and bioactive potential of watermelon seeds: a pathway to sustainable food and health innovation. Sustainable Food Technology. 2025;3:375-395. Available from: https://doi.org/10.1039/D4FB00335G
38. Manikandan P, Vinothini G, Vidya Priyadarsini R, Prathiba D, Nagini S. Eugenol inhibits cell proliferation via NF-κB suppression in a rat model of gastric carcinogenesis induced by MNNG. Investigational New Drugs. 2011;29:110-117. Available from: https://doi.org/10.1007/s10637-009-9345-2
39. Rodrigues TG, Fernandes Jr A, Sousa JPB, Bastos JK, Sforcin JM. In vitro and in vivo effects of clove on pro-inflammatory cytokines production by macrophages. Natural Product Research. 2009;23(4):319-326. Available from: https://doi.org/10.1080/14786410802242679
40. Shi X, Zhang W, Bao X, Liu X, Yang M, Yin C. Eugenol modulates the NOD1-NF-κB signaling pathway via targeting NF-κB protein in triple-negative breast cancer cells. Frontiers in Endocrinology. 2023;14:1136067. Available from: https://doi.org/10.3389/fendo.2023.1136067
41. Zari AT, Zari TA, Hakeem KR. Anticancer properties of eugenol: A review. Molecules. 2021;26(23):7407. Available from: https://doi.org/10.3390/molecules26237407
42. Idoko AS, Abdullahi A, Maibulangu BM, Nura L, Imam NU, Bonomi ZM, et al. Allium sativum and Curcuma longa powder protect against hepatotoxic and nephrotoxic effect of high fructose diet. FUOYE Journal of Pure and Applied Sciences. 2022;7(8):60. Available from: https://doi.org/10.55518/fjpas.JGKT1690
43. Bosland MC, Schlicht MJ, Horton L, McCormick DL. The MNU plus testosterone rat model of prostate carcinogenesis. Toxicologic Pathology. 2022;50(4):478-496. Available from: https://doi.org/10.1177/01926233221096345
44. Ibrahim AY, Mahmoud MG, Asker MS, Youness ER, El-Newary SA. Acidic exo-polysaccharide obtained from Bacillus sp. NRC5 attenuates testosterone-DMBA-induced prostate cancer in rats via inhibition of 5 α-reductase and Na+/K+ ATPase activity mechanisms. Current Microbiology. 2023;80(1):8. Available from: https://doi.org/10.1007/s00284-022-03098-8
45. Strate T, Mann O, Kleinhans H, Rusani S, Schneider C, Yekebas E, et al. Microcirculatory function and tissue damage is improved after therapeutic injection of bovine hemoglobin in severe acute rodent pancreatitis. Pancreas. 2005;30(3):254-259. Available from: https://doi.org/10.1097/01.mpa.0000157481.22155.2d
46. Eke R, Ejiofor E, Oyedemi S, Onoja S, Omeh N. Evaluation of nutritional composition of Citrullus lanatus Linn. (watermelon) seed and biochemical assessment of the seed oil in rats. Journal of Food Biochemistry. 2021;45(6):13763. Available from: https://doi.org/10.1111/jfbc.13763
47. Shaban NZ, El-Kot SM, Awad OM, Hafez AM, Fouad GM. The antioxidant and anti-inflammatory effects of Carica papaya Linn. seeds extract on CCl₄-induced liver injury in male rats. BMC Complementary Medicine and Therapies. 2021;21:1-15. Available from: https://doi.org/10.1186/s12906-021-03479-9
48. Gao X, Luo F, Zhao H. Cloves regulate Na⁺‐K⁺‐ATPase to exert antioxidant effect and inhibit UVB light‐induced skin damage in mice. Oxidative Medicine and Cellular Longevity. 2021;2021(1):5197919. Available from: https://doi.org/10.1155/2021/5197919
49. Sharma A, Sharma R, Sharma M, Kumar M, Barbhai MD, Lorenzo JM, et al. Carica papaya L. leaves: deciphering its antioxidant bioactives, biological activities, innovative products, and safety aspects. Oxidative Medicine and Cellular Longevity. 2022;2022(1):2451733. Available from: https://doi.org/10.1155/2022/2451733
50. Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, Clements J, Hutmacher DW. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. International Journal of Molecular Sciences. 2013;14(7):14620-14646. Available from: https://doi.org/10.3390/ijms140714620
51. Oseni OA, Odesanmi OE, Oladele FC. Antioxidative and antidiabetic activities of watermelon (Citrullus lanatus) juice on oxidative stress in alloxan-induced diabetic male Wistar albino rats. Nigerian Medical Journal. 2015;56(4):272-277. Available from: https://doi.org/10.9734/JALSI/2017/33815
52. Zhang L, Guo J, Zhang Q, Zhou W, Li J, Yin J, et al. Flutamide induces hepatic cell death and mitochondrial dysfunction via inhibition of Nrf2‐mediated heme oxygenase‐1. Oxidative Medicine and Cellular Longevity. 2018;2018:8017073. Available from: https://doi.org/10.1155/2018/8017073
53. Salla S, Sunkara R, Walker LT, Verghese M. Antioxidant and apoptotic activity of papaya peel extracts in HepG2 cells. Food and Nutrition Sciences. 2016;7(6):485-494. Available from: https://doi.org/10.4236/fns.2016.76050
54. Padhy I, Paul P, Sharma T, Banerjee S, Mondal A. Molecular mechanisms of action of eugenol in cancer: recent trends and advancement. Life. 2022;12(11):1795. Available from: https://doi.org/10.3390/life12111795
55. Alwan S, Hatem T, Abid H. Molecular mechanisms of eugenol as an antitumour bioactive compound: A comprehensive review. In BIO Web of Conferences. Vol. 125. EDP Sciences; 2024. p. 03007. Available from: https://doi.org/10.1051/bioconf/202412503007
56. Cronauer MV, Ince Y, Engers R, Rinnab L, Weidemann W, Suschek CV, et al. Nitric oxide-mediated inhibition of androgen receptor activity: possible implications for prostate cancer progression. Oncogene. 2007;26(13):1875-1884. Available from: https://doi.org/10.1038/sj.onc.1209984
57. Prakash P, Verma S, Gupta S. Risk factors influencing chronic inflammation in neoplastic transition to prostate cancer. Journal of Translational Genetics and Genomics. 2024;8(4):312-327. Available from: https://doi.org/10.20517/jtgg.2024.52
58. Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(10):2004-2009. Available from: https://doi.org/10.1161/01.ATV.17.10.2004
59. Soni Y, Softness K, Arora H, Ramasamy R. The Yin Yang role of nitric oxide in prostate cancer. American Journal of Men’s Health. 2020;14(1):1557988320903191. Available from: https://doi.org/10.1177/1557988320903191
60. Asgary S, Soltani R, Daraei F, Salehizadeh L, Vaseghi G, Sarrafzadegan N. The effect of lycopene on serum level of cardiac biomarkers in patients undergoing elective percutaneous coronary intervention: A randomized controlled clinical trial. ARYA Atherosclerosis. 2021;17(1):1. Available from: https://doi.org/10.22122/arya.v17i0.2194
61. Assar EA, Vidalle MC, Chopra M, Hafizi S. Lycopene acts through inhibition of IκB kinase to suppress NF-κB signaling in human prostate and breast cancer cells. Tumor Biology. 2016; 37:9375-9385. Available from: https://doi.org/10.1007/s13277-016-4798-3
62. Cao Y, Lai KM, Fu KC, Kuo CL, Tan YJ, Yu L, et al. Dual functionality of papaya leaf extracts: anti-coronavirus activity and anti-inflammation mechanism. Foods. 2024;13(20):3274. Available from: https://doi.org/10.3390/foods13203274
63. Gurusmatika S, Ishida M, Nishi K, Sugahara T. Exploring the anti-inflammatory effect of clove water extract in lipopolysaccharide-stimulated RAW264.7 cells and mouse peritoneal macrophages. Journal of Food Bioactives. 2024;25. Available from: https://doi.org/10.31665/JFB.2024.18373
64. Uyanga VA, Wang M, Tong T, Zhao J, Wang X, Jiao H, et al. L-citrulline influences the body temperature, heat shock response and nitric oxide regeneration of broilers under thermoneutral and heat stress condition. Frontiers in Physiology. 2021; 11:671691. Available from: https://doi.org/10.3389/fphys.2021.671691
65. Cai Y, Lee YF, Li G, Liu S, Bao BY, Huang J, et al. A new prostate cancer therapeutic approach: combination of androgen ablation with COX‐2 inhibitor. International Journal of Cancer. 2008;123(1):195-201. Available from: https://doi.org/10.1530/ERC-10-0339
66. Alwan S, Hatem T, Abid H. Molecular mechanisms of eugenol as an antitumour bioactive compound: A comprehensive review. In BIO Web of Conferences. Vol. 125. EDP Sciences; 2024. p. 03007. Available from: https://doi.org/10.1051/bioconf/202412503007
67. Wu CT, Chen WC, Lin PY, Liao SK, Chen MF. Androgen deprivation modulates the inflammatory response induced by irradiation. BMC Cancer. 2009; 9:92. Available from: https://doi.org/10.1186/1471-2407-9-92
68. Álvarez-Maestro M, Eguibar A, Chanca P, Klett-Mingo M, Gómez Rivas J, Buño-Soto A, et al. Androgen deprivation therapy in patients with prostate cancer increases serum levels of thromboxane A2: cardiovascular implications. Frontiers in Cardiovascular Medicine. 2021; 8:653126. Available from: https://doi.org/10.3389/fcvm.2021.653126
69. Nisar A, Jagtap S, Vyavahare S, Deshpande M, Harsulkar A, Ranjekar P, et al. Phytochemicals in the treatment of inflammation-associated diseases: the journey from preclinical trials to clinical practice. Frontiers in Pharmacology. 2023; 14:1177050. Available from: https://doi.org/10.3389/fphar.2023.1177050
70. Rajgopal A, Rebhun JF, Burns CR, Scholten JD, Balles JA, Fast DJ. Immunomodulatory effects of Lippia sidoides extract: induction of IL-10 through cAMP and p38 MAPK-dependent mechanisms. Journal of Medicinal Food. 2015;18(3):370-377. Available from: https://doi.org/10.1089/jmf.2014.0096
71. Saraiva M, Vieira P, O’Garra A. Biology and therapeutic potential of interleukin-10. Journal of Experimental Medicine. 2020;217(1):e20190418. Available from: https://doi.org/10.1084/jem.20190418
72. Jin Q, Liu T, Qiao Y, Liu D, Yang L, Mao H, et al. Oxidative stress and inflammation in diabetic nephropathy: role of polyphenols. Frontiers in Immunology. 2023; 14:1185317. Available from: https://doi.org/10.3389/fimmu.2023.1185317
73. Rapa SF, Di Iorio BR, Campiglia P, Heidland A, Marzocco S. Inflammation and oxidative stress in chronic kidney disease—potential therapeutic role of minerals, vitamins and plant-derived metabolites. International Journal of Molecular Sciences. 2020;21(1):263. Available from: https://doi.org/10.3390/ijms21010263
74. Yanez M, Blanchette J, Jabbarzadeh E. Modulation of inflammatory response to implanted biomaterials using natural compounds. Current Pharmaceutical Design. 2017;23(41):6347-6357. Available from: https://doi.org/10.2174/1381612823666170510124348
75. Pal P, Banerjee ER. Exploring the effect of phytochemical extract (Benincasa sp.) on the inflammatory mediators and regulatory pathways of acute cisplatin nephrotoxicity in pre-clinical mouse model. Biomedical Research and Therapy. 2024;11(6):6494-6510. Available from: https://doi.org/10.15419/bmrat.v11i6.896
76. Bohn T, McDougall GJ, Alegría A, Alminger M, Arrigoni E, Aura AM, et al. Mind the gap—Deficits in our knowledge of aspects impacting the bioavailability of phytochemicals and their metabolites—A position paper focusing on carotenoids and polyphenols. Molecular Nutrition & Food Research. 2015;59(7):1307-1323. Available from: https://doi.org/10.1002/mnfr.201400745
77. Brodzicka A, Galanty A, Paśko P. Modulation of multidrug resistance transporters by food components and dietary supplements: implications for cancer therapy efficacy and safety. Current Issues in Molecular Biology. 2024;46(9):9686-9706. Available from: https://doi.org/10.3390/cimb46090576
78. Gómez-Garduño J, León-Rodríguez R, Alemón-Medina R, Pérez-Guillé BE, Soriano-Rosales RE, González-Ortiz A, et al. Phytochemicals that interfere with drug metabolism and transport, modifying plasma concentration in humans and animals. Dose-Response. 2022;20(3):15593258221120485. Available from: https://doi.org/10.1177/15593258221120485
79. Rathee S, Patil UK, Jain SK. Exploring the potential of dietary phytochemicals in cancer prevention: A comprehensive review. Journal of Exploratory Research in Pharmacology. 2024;9(1):34-47. Available from: https://doi.org/10.14218/JERP.2023.00050
80. Gong J, Tu W, Liu J, Tian D. Hepatocytes: A key role in liver inflammation. Frontiers in Immunology. 2023; 13:1083780. Available from: https://doi.org/10.3389/fimmu.2022.1083780
81. Wu S, Wang X, Xing W, Li F, Liang M, Li K, et al. An update on animal models of liver fibrosis. Frontiers in Medicine. 2023; 10:1160053. Available from: https://doi.org/10.3389/fmed.2023.1160053
82. Choudhari AS, Mandave PC, Deshpande M, Ranjekar P, Prakash O. Phytochemicals in cancer treatment: From preclinical studies to clinical practice. Frontiers in Pharmacology. 2020; 10:1614. Available from: https://doi.org/10.3389/fphar.2019.01614